Meet volunteers from Affiliates/Chapters and Women in Pharma®.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Meet ISPE Emerging Leader volunteers and the 2022 Europe Hackathon Committee.
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are recent technical articles about quality, including global commissioning, qualification, and validation; data for verification; and addressing N-nitrosodimethylamine (NDMA) impurities.
Meet ISPE volunteers from our Asia Pacific Affiliates.
Meet ISPE volunteers participating in Publishing, Guidance Documents, Technical Communities, & Regulatory.
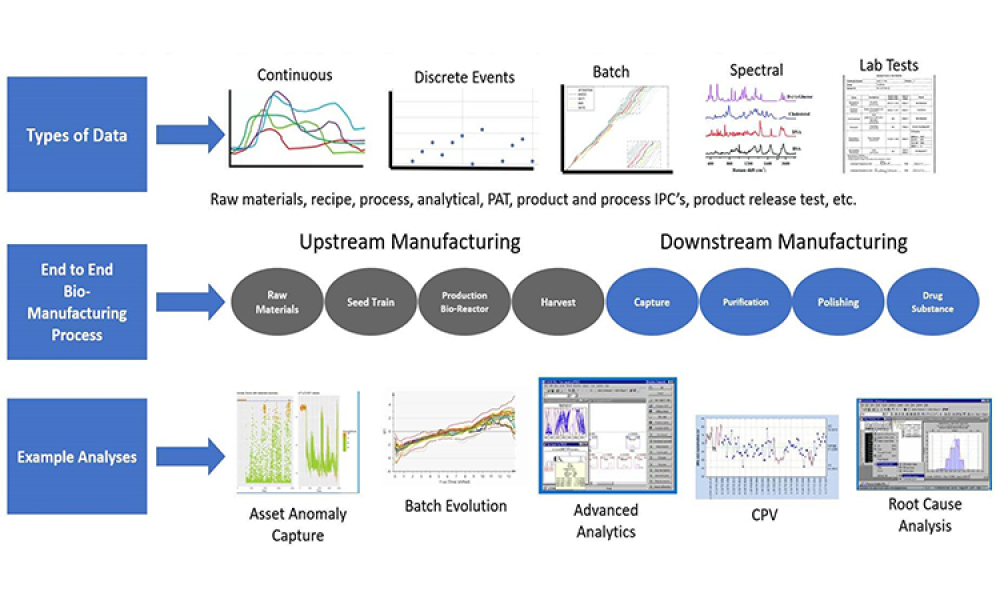
Digital transformation has been growing in importance and urgency as the biopharma and biotech industry, manufacturers, and service providers seek to accelerate build-out of facilities and manufacturing processes in support of advanced biotherapeutics.
The Covid pandemic has pushed all companies to accelerate their digitization plans and ramp up adoption of emerging technologies. For regulated companies like Pharma, which operated in secure offices and fortified offshore development centers, this has also meant new ways of working and additional challenges in cybersecurity. Furthermore, with the need for agility in cases like developing the...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from March 2022. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
The 2022 ISPE Biotechnology Conference on June 28-30 in Boston, MA and virtually will offer attendees several unique perspectives and case studies across several separate content areas.
Single-use plastics are critical for healthcare, life sciences laboratories, and bioprocessing. They have many benefits over other materials and their use is increasing. In particular, single-use technologies for bioprocessing decrease costs, reduce water and energy usage, and increase efficiency and scalability. However, most of these high-value, virgin materials end up in landfills and...
As Industry professionals devoted to developing and inspiring the love of STEM, it has always been our goal to share our knowledge and expertise with those who may be curious about the diverse roles available in the growing biotech industry, while also helping them to grow their professional networks to assist them with their future educational and career goals. Many of us began our own...
As we emerge from a global pandemic that has impacted all aspects of life – both personal and professional, we, in the pharmaceutical industry, should take the opportunity to reflect, learn, and look forward to the exciting changes and innovations within our industry. The past two years have seen unprecedented innovations in the development and manufacture of products that provide the promise...
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are articles addressing an issue that became a headline topic during the pandemic—and one that the industry continues to address: drug shortages and the supply chain. There are many questions about these issues: these articles present some strategies for addressing the challenges.
What is the current state of your organization’s Change Management (CM) System? The effective management of change throughout the product lifecycle enables quality improvement and is critical to patient safety, supply reliability, as well as operational effectiveness and efficiency.
The 2022 Planning Committee is delighted to welcome attendance and registration for the fast-approaching ISPE Biotechnology Conference, taking place 28-30 June in Boston, Massachusetts and virtually.
The life sciences industry is in the middle of a significant construction boom, partly fueled by research and manufacturing of vaccines and therapies to fight the COVID-19 virus and its variants. There are hundreds of new projects under construction, in various stages of planning or just completed. The developments run the gamut from production facilities to office space to laboratories for...
Microbial quality encompasses sterility assurance—and more, as discussed by Joyce Hansen, Vice President of J&J Microbiological Quality and Sterility Assurance at Johnson & Johnson in the keynote at the Plenary Session, “Microbiology Quality and Cultivating Emerging Leaders” that kicked off Day 2 of the 2022 ISPE Aseptic Conference.
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from February 2022. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.