ATMPs, mRNA, Digital Transformation, & More
The 2022 Planning Committee is delighted to welcome attendance and registration for the fast-approaching ISPE Biotechnology Conference, taking place 28-30 June in Boston, Massachusetts and virtually.

The 2022 Planning Committee is delighted to welcome attendance and registration for the fast-approaching ISPE Biotechnology Conference, taking place 28-30 June in Boston, Massachusetts and virtually.
For more than seven years, this event has included participation from biopharmaceutical industry professionals, regulators, and service providers, providing knowledge and leading-edge technical know-how that has become recognized as the premier forum for industry leaders to share the latest research in bioprocess R&D, scale-up, quality, analytics and commercial manufacturing. As we navigate the new realities of conferences and gatherings, you can count on ISPE’s commitment to deliver quality content and facilitate networking opportunities to support the research, drug substance and product manufacturing goals of this industry.
The new hybrid event model presenting ATMPs/C>s, mRNAs, mAbs, E2E continuous manufacturing/process intensification, Pharma 4.0™/digital integration, sustainability, risk mitigation and management and other aspect of this industry gives you the option to experience the conference in-person or virtually.
The biopharmaceutical industry, in particular mAb therapeutics, is now considered matured, with multiple mAbs and biosimilars pursuing the same indications. With increasing competition, companies are focusing on cost savings. Process intensification via continuous processing fills this urgent need. However, despite the promise of reduced COGS, the industry is still slow to adopt continuous processing at clinical or manufacturing scale. This conference dives into the challenges and successes in implementing continuous processes, by inviting scientists to share case studies of intensified perfusion process, continuous downstream strategies, and integrated up- and downstream approaches for mAbs and non-mAbs. In addition, discussing new trends and supporting technologies such as PAT, real-time process monitoring, modeling, and automation.
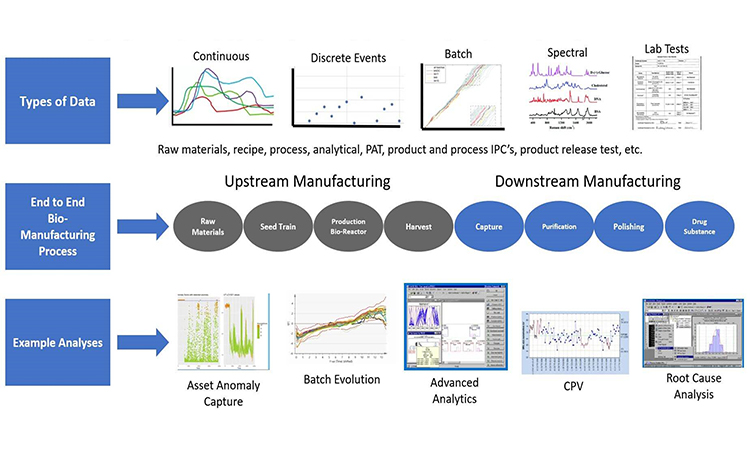
The process of manufacturing biopharmaceuticals or their precursors has experienced persistent, yet discontinuous technological progress ever since the release of the first blockbuster biologics. Major technological advances have taken place in various aspects of bioproduction, from cell biology and gene integration to bioprocess media and chromatographic technologies.
However, due to regulatory, cost, and other risk factors, it can be difficult to implement new bioprocess technologies. New technologies are typically introduced early in the product development lifecycle and can take months or even years to be implemented for manufacturing’s benefit. Even in the earliest stages of a new product development cycle, there is a propensity to design bioprocesses strictly on well-known technologies, which is sometimes heralded as a “platform process” or “modality”– in the sense, if it isn’t broken, don’t fix it approach.
Conference attendees will have the opportunity to explore:
Presentations, panel discussions, and interactive sessions will feature industry insights, case studies, and lessons learned on:
Overall, I am very proud to be a part of the conference committee and of the comprehensive technical program we can offer. We are all looking forward to seeing you—hopefully in person!
In the pharmaceutical industry, disaster recovery is not just about protecting business continuity; it is about safeguarding patient safety, data integrity, and the entire supply chain. The increasing complexity of global pharmaceutical operations exposes companies to a broad spectrum of risks, ranging from man-made disasters such as geopolitical conflicts to natural disasters that can cripple...
ISPE’s Q12 Implementation team, a working group under the auspices of ISPE's Product Quality Lifecycle Implementation (PQLI)® committee, continued their series of training events with a well-attended course delivered to Singapore’s Health Sciences Authority (HSA) in November 2024.