Biotech is adapting and evolving to have more automation, digitalization, and we need to be ready for the new challenges. Join us at the 2022 ISPE Facility of the Future Conference...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Over the past decade, companies worldwide have faced increased scrutiny from consumers, investors, and regulators over how they treat the environment. They are questioned about where and how they source raw materials, the packaging they use, power consumption levels, and waste generated. Many companies understand these concerns and have taken active steps to be sustainable. Building industry...
ISPE published the 2nd Edition of the ISPE Good Practice Guide: Controlled Temperature Chambers – Commissioning and Qualification, Mapping and Monitoring in...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from December 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
I’m from the past, but to some degree, we all are.
A number of years ago, I received my Bachelor’s degree in mechanical engineering and a desire to make the world a better place. I joined the pharmaceutical industry with that goal in mind. 30 years later, I have that same desire, but the industry looks significantly different.
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are Online Exclusives. These are PE articles published exclusively on the website; you can always find them here. Here is a selection from the first two years of Online Exclusives on...
Continuous Bioprocessing Platform
Continuous processing is not new to the pharmaceutical industry. Today, the majority of major pharmaceutical companies at least consider continuous processes when developing new small-molecule entities. In contrast, the biopharmaceutical industry has been slow to adopt continuous platforms, particularly related to high volume processes. This...
The ISPE Aseptic Conference is back and in-person for 2022! We have a great line-up of speakers during the two-day conference, and of course, a great group for the Interactive Regulatory Panel. The...
When most people hear “Facility of the Future,” they immediately think about robots, automation, virtual reality, or logging in with a Facebook account, security issues aside. However, when I hear “Facility of the Future,” I think of lean manufacturing and flexibility. The majority of my career has been on the design side, where I focused on building facilities. Historically, the projects I...
Knowledge Management (KM) is a discipline (akin to Quality Risk Management (QRM) or Lean Six Sigma) that focuses on how organizations create, manage, use, and share knowledge. The publication of ICH Q10 in 2008 saw the formal designation of KM as a key enabler for an effective Pharmaceutical Quality System (PQS), along with QRM. Since then, the industry has acknowledged the...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from November 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
A thought-provoking session held during the 2021 ISPE Annual Meeting & Expo provided insight into how the Overall Quality Summary can provide an ideal opportunity to provide a...
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are some great reading selections for the holidays! This year’s holiday topic: biopharma, cell and gene therapies, and ATMPs. That topic is the theme for the November-December 2021 issue , and here are some earlier articles on these important and timely topics.
ISPE starts 2022 with a bang, with the 31st Annual Face-to-Face 2022 ISPE Aseptic Conference, March 14-15, offering attendees several unique perspectives and case studies across various...
Competitive advantage and sustained business results depend on leaders building and maintaining a strong culture. This has been reinforced by the increased challenges in attracting and retaining talent. The FDA is also placing significant focus on Quality Management Maturity. But how do leaders know if they are on track? Assessing leadership systems is often overlooked or much more difficult...
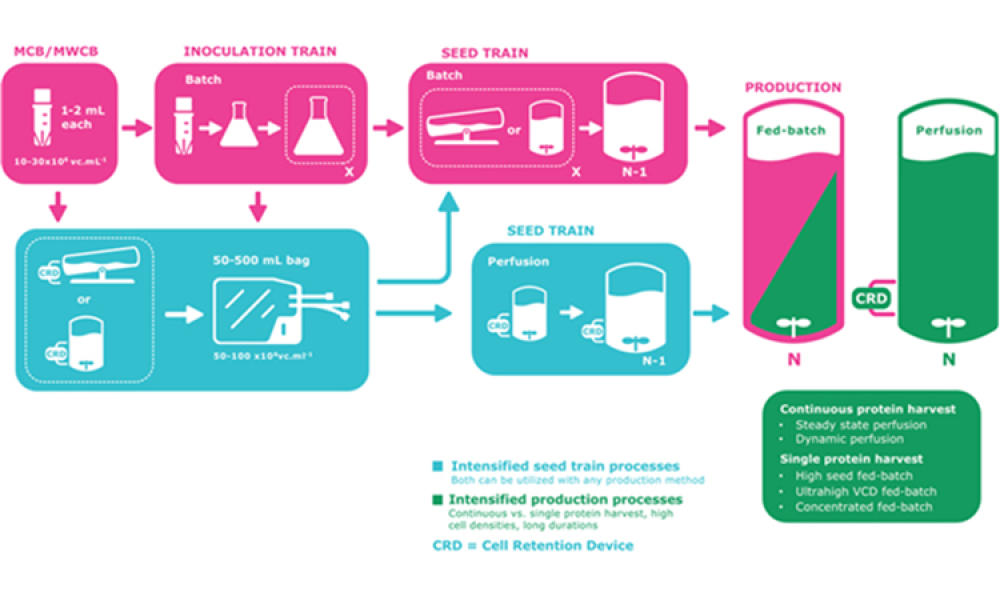
Vaccine Manufacturing
The industry is experiencing an acceleration in the development and approval of novel prophylactic and therapeutic vaccine modalities. Concurrently, many biopharmaceuticals are experiencing the benefits of manufacturing process intensification (PI). Describing such advances for cell-culture based processes is challenging in the field of vaccinology because of...
It is a great honour and pleasure for me as Chair of the ISPE Board of Directors and Chair of the 2022 ISPE Aseptic Conference Program Committee to give you an overview of the upcoming conference including information about the knowledge that will be shared, a look at some of the speakers, and details about a few of the networking opportunities.
Volunteers are the lifeblood of ISPE. Along with staff, they implement the global mission of the Society and produce essential content that benefits the life sciences industry. ISPE values our volunteers’ time and the commitment they make to sharing their knowledge and talents for the good of others. There are numerous ways to get involved with ISPE from serving on a committee to reviewing a...