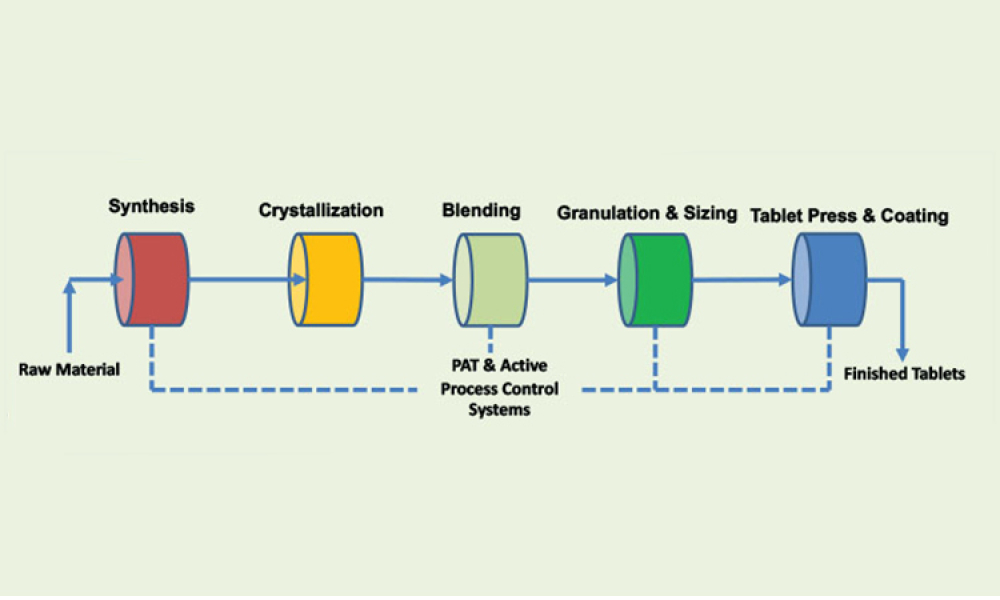
Process Analytical Technology (PAT) and lifecycle control strategy are umbrella terms covering a range of essential components for other innovative initiatives, such as quality by design, real-time release, and continuous manufacturing.
Guidance Documents
Knowledge Management (1)
Lifecycle Management (3)
Process Analytical Technology (4)
Community Discussions
Community Discussions
Sep 18, 2024
Knowledge Management
Process Analytical Technology
Webinars
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Quality Risk Management (QRM) Workshop Training Course
This interactive advanced workshop uses case studies to provide practical tools and techniques to identify solutions for applying QRM principles to current challenges and provides hands-on experience with preparing for and facilitating risk assessments.
Implementing Process Analytical Technology Training Course
This course is designed to help pharmaceutical manufacturing professionals chart a new course for innovation based on PAT. The course provides an overview to the tools and principles outlined in the FDA guidance, PAT - A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance.
GAMP® 5 GxP Process Control Training Course
This highly interactive classroom course describes how the GAMP Good Practice Guide: A Risk-Based Approach to Compliant GxP Process Control Systems, and GAMP RDI Good Practice Guide: Data Integrity - Manufacturing Records may be applied to achieve process control systems that are fit for intended use, incorporate data integrity and meet current regulatory requirements.
White Papers
January / February 2025
GAMP® is indispensable for safeguarding the safety, quality, and compliance of pharmaceutical…
November / December 2024
Plastic Process Waste in Biopharmaceutical Manufacturing Cover: This article presents a…
September / October 2024
PIC/S in Latin America: Harmonization of cGMP Procedures Cover: This article offers an overview of…