Thomas Hartman, ISPE’s President & CEO and active ISPE Member for over 20 years, speaks with us on his favorite ISPE memories, his real-world examples on the benefits of ISPE, and why volunteering is so important. Join us as we get to know Thomas a little more.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Dr. Randy Perez retired from the position of Director of Information Governance and Management for Novartis Pharmaceuticals in 2015. During his 32-year tenure at Novartis his responsibilities included a wide range of IT Compliance issues, such as GxP, Sarbanes-Oaxley, and data privacy. He served on several global teams dealing with computer systems compliance issues and authored many of the...
ISPE continues to work with a group of industry associations to assist the European Union (EU) and the European Medicines Agency (EMA) with implementation of revision of Annex 1, Manufacture of Sterile Products....
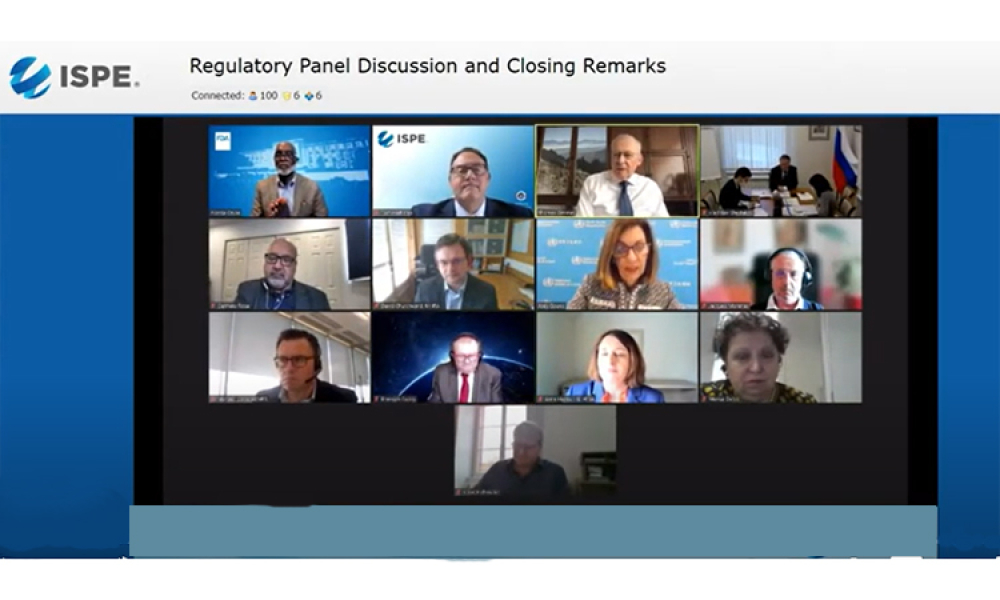
This virtual ISPE Global Pharmaceutical Regulatory Summit, held on 28 April 2021, brought together eleven regulators from different parts of the world to discuss how their approach to GMP inspections have adapted to the COVID-19 pandemic. Such a large number of regulators at a single forum is unprecedented for ISPE.
As a young Science graduate, I couldn’t think of anything worse than being stuck at the same desk, day in / day out, in a stuffy office, doing the same thing day after day, just to earn a living. There had to be more to life, right? I wanted to travel the world, see new places, not be defined by what I do or how I look, and most definitely not be pigeonholed due to my career choice or hair...
The main change can be seen on the imports of IMPs from EU/EEA to Great Britain starting 01 January 2022. Sponsors of United Kingdom Clinical trials will need to appoint a United Kingdom Manufacture & Importation Authorization (MIA) (IMP) holder who will be responsible for implementing an oversight process to confirm each batch has been certified by a Qualified Person before its release to...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from April 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
While Pharmaceutical development and manufacturing have focused for years on robust quality management systems (QMS), the application of quality risk management (QRM) applying digital tools and advances are critical to future success. Coupling controls, artificial intelligence, and associated platforms with basic QMS and QRM affords a great opportunity for the pharmaceutical industry.
Case studies demonstrating the link between knowledge and risk in technology transfer and commercial manufacture
Economic Operators Registration and Identification (EORI) Number
Customs procedures also imply having an Importer of Record liable at destination as well as having each party, exporter, and importer, registered, and identified with its own EORI (from the Country it is based at). An XI EORI number is required to move goods between NI and non-EU Countries (which comprise Great...
Featured in this edition of the Pharmaceutical Engineering Online Reading Roundup are the most read online articles during April 2021. Learn more about understanding cleanliness classifications, steam sterilization, and more from what visitors to the PE Online site were reading last month.
Quality is the strongest force multiplier for any organization’s growth. Attaining higher quality levels has a multi-dimensional effect throughout any organization. Creating and sustaining a passion for quality brings higher levels of responsiveness, confidence and flexibility to organization wide manufacturing operations. Overall quality and manufacturing data as represented by effective...
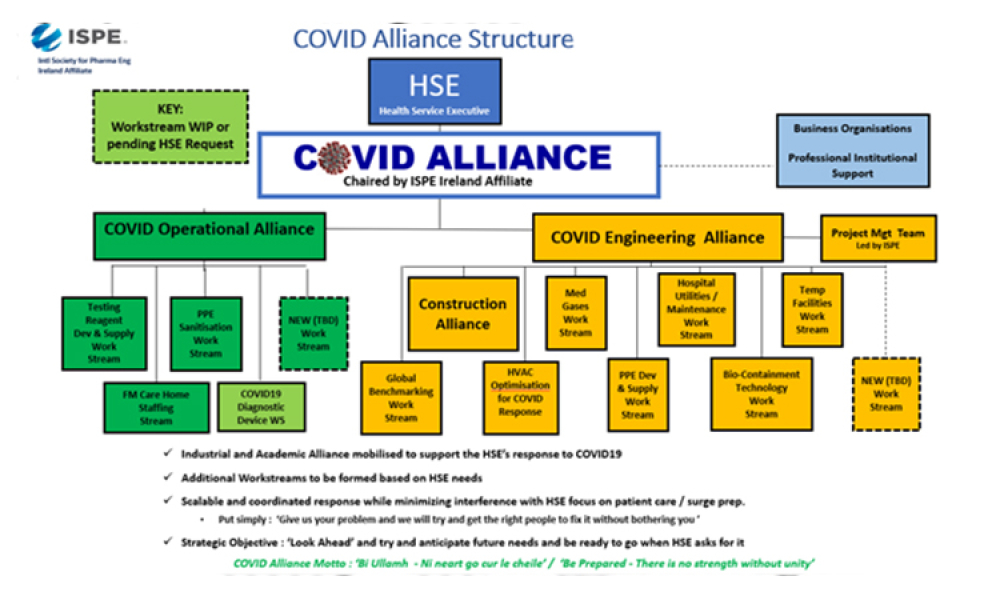
Early in March 2020, many around the world viewed the sight of collapsing intensive care units in Northern Italy, overwhelmed by COVID19, with great anxiety and we wondered how we might address the inevitable arrival of the virus at our doorsteps. This concern led to a home-grown initiative in Ireland that brought together representatives of health, commercial and academic organisations...
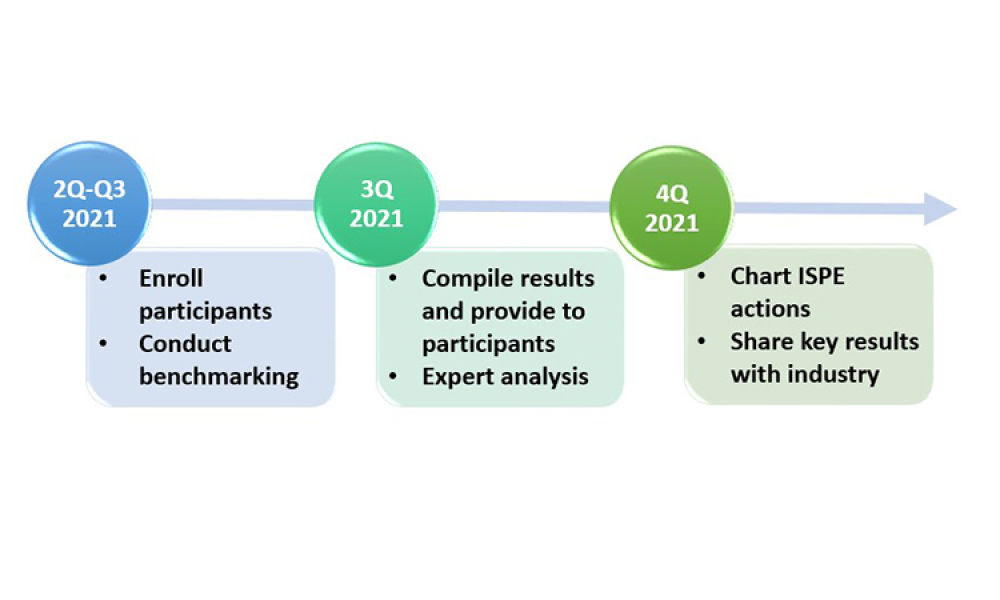
ISPE is pleased to announce that its Drug Shortages Initiative Task Team is launching a new phase of work to focus on business continuity planning for the prevention of drug shortages. We invite your company to participate. The first activity is a benchmarking study that will launch in May 2021 and be used to assess best practices and industry accomplishments in the area of business continuity...
You are an active and engaged ISPE Member! You sit on the ISPE Board of Directors, the ISPE Foundation Board, and the Steering Committee of Women in Pharma®. Can you share...
Prathiba Sampath, PMPFor me, both personally and professionally, 2020 started off as an incredible year. My husband and I were expecting the arrival of our first child in April. At work, I was being entrusted with increasing responsibilities and being groomed for a promotion, while having an incredible learning journey. I’d taken the lead on the development project for a new manufacturing...
The UK Entered a New Relationship with the European Union on 1 Jan 2021
The United Kingdom (UK) (England, Northern Ireland, Scotland and Whales) is no longer part of the European Union (EU) Customs Union. Simply put, it means movement of goods between the UK and the EU are now to be an import and export trade transaction. Multiple industries have been impacted, including...