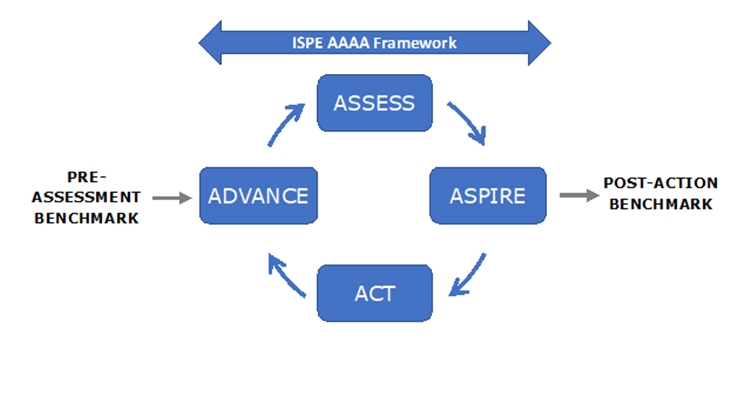
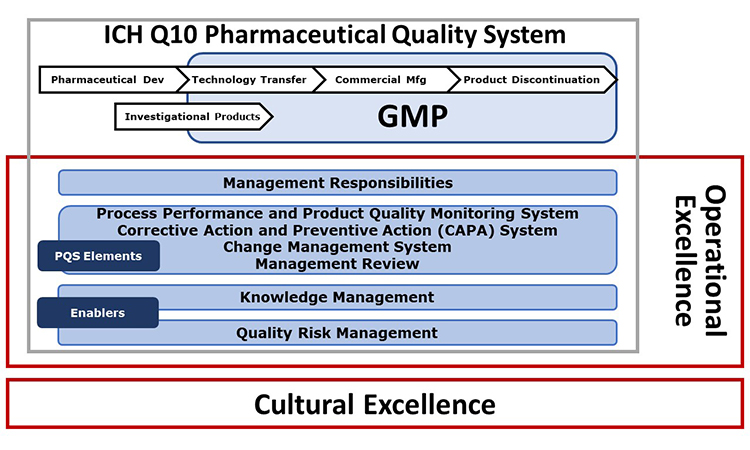
The extended model shown in Figure 1 indicates other important initiatives. The University of St. Gallen has worked over many years in the field of Operational Excellence (OPEX) and in recent years has collaborated with ISPE in development of the APQ Program. This fruitful collaboration has led to incorporation of the St. Gallen concise, user-friendly OPEX benchmarking module as an option in the APQ Program.
 ISPE has had a very active Knowledge Management initiative with the team recently publishing the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry. This Guide will be an excellent complementary resource to the ISPE APQ Program.
ISPE has had a very active Knowledge Management initiative with the team recently publishing the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry. This Guide will be an excellent complementary resource to the ISPE APQ Program.
ISPE has continued its Cultural Excellence work leading to a supplement to the 2017 Cultural Excellence report being produced on Reward and Recognition and best practices for conducting a GEMBA Walk. There are many references in the APQ Guides to potential cultural excellence assessment and improvement activities, which are practical and realistic. There is the prospect that all this Cultural Excellence work will be consolidated as another Guide in the APQ Guide Series.
FDA has also shown considerable interest in quality management maturity. In 2018 and 2019, the FDA drew from St. Gallen research as the agency started to discuss the concept of quality maturity in public presentations leading to an extensive discussion on quality management maturity in the FDA report, Drug Shortages: Root Causes and Potential Solutions.
In fall 2020 FDA announced a quality management pilot program.,, Through this pilot program, a third-party contractor identified by the FDA will conduct an onsite assessment of a facility’s quality management system, accompanied by FDA staff. The Agency will gain insight from the results of the Quality Management Maturity (QMM) assessments to inform the development of a framework for conducting QMM assessments of manufacturers and a rating system that will incentivize industry investments in quality management maturity. Participation is voluntary and the participating sites will be able to use assessment information in their continual improvement efforts.
ISPE believes that the APQ AAAA framework should assist with the challenge of assessing quality management maturity.
Conclusion
Significant progress has been made with the ISPE APQ quality maturity management program, Advancing Pharmaceutical Quality with the real prospect that the complete program will be available in mid 2022.
Register for Webinar
 ISPE’s quality management maturity program, Advancing Pharmaceutical Quality (APQ) has progressed significantly. The first Good Practice Guide in a series, Corrective Action, Preventive Action (CAPA) was published in December 2020. To assist practitioners, a complimentary webinar, Advancing Pharmaceutical Quality, Corrective Action/Preventive Action (CAPA), to explain the CAPA APQ program is scheduled for Wednesday, 26 May 2021 at 11:00 EST. In addition to ISPE experts, there will be participation from the USFDA and the University of St. Gallen, Switzerland.
ISPE’s quality management maturity program, Advancing Pharmaceutical Quality (APQ) has progressed significantly. The first Good Practice Guide in a series, Corrective Action, Preventive Action (CAPA) was published in December 2020. To assist practitioners, a complimentary webinar, Advancing Pharmaceutical Quality, Corrective Action/Preventive Action (CAPA), to explain the CAPA APQ program is scheduled for Wednesday, 26 May 2021 at 11:00 EST. In addition to ISPE experts, there will be participation from the USFDA and the University of St. Gallen, Switzerland.