Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
The environmental impact of “single-use plastics” has become a hot topic of societal and political debate. Despite the many technological advantages and innovations that plastic has enabled, it is now under closer scrutiny, especially those massively used in consumer market (plastic bottles, cutlery, straws, packaging, etc.).
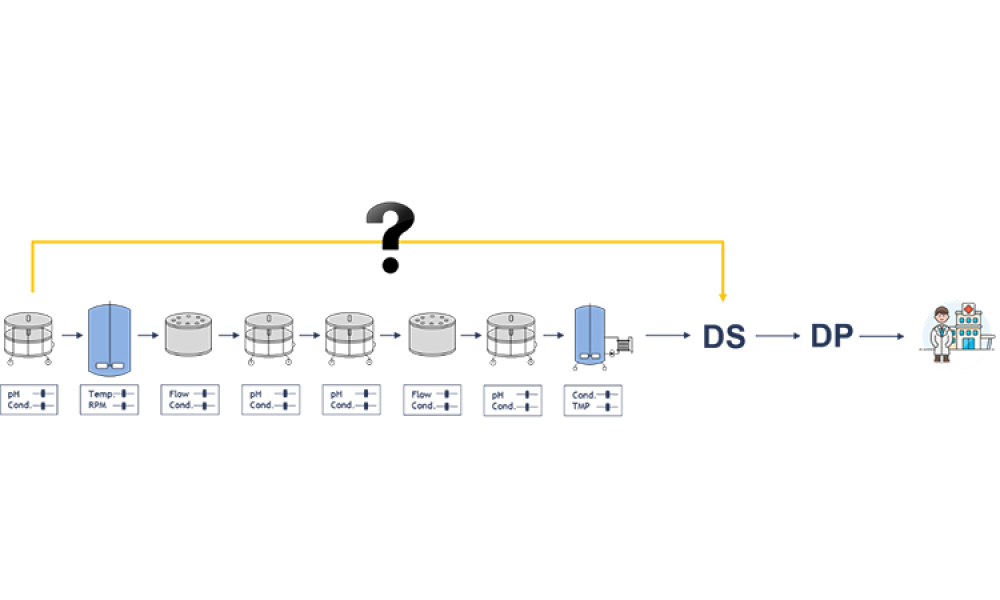
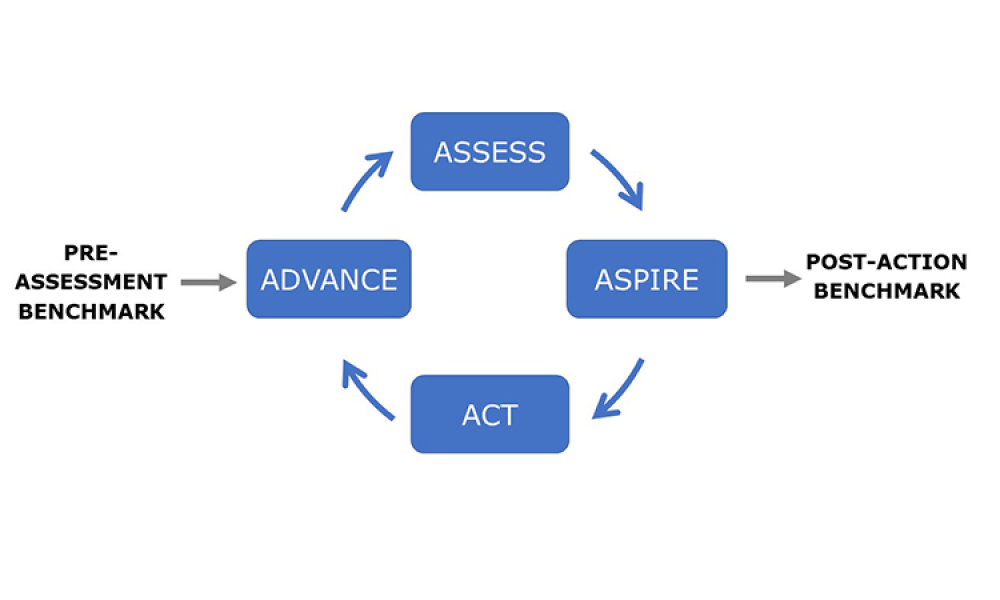
Process validation (PV) aims at reassuring a manufacturer of constant product quality. Moreover, it is a regulatory requirement to achieve licensure of a pharmaceutical product Failing to efficiently plan and execute activities here leads to increased time-to-market. Statistics play a pivotal role here, as is shown by the fact that in the 22 pages of the latest FDA guidance document on process...
The needs of patients must always be at the forefront of what we do.
Wow! You have been with the Society for 40 years! Can you give me a brief summary of your involvement with ISPE and share some of the more interesting experiences and successes you have had with the Society during your lengthy tenure?
Hope everyone is settling into their new reality and staying safe. Your ISPE Annual meeting education organizers have been hard at work setting up a fantastic program to be held in the fall. Check it out on line and plan on joining with colleagues and experts around the world to review and discuss the latest developments in the sector.
The individual stories of patients—and their families—are welcome reminders to the pharmaceutical industry about just how meaningful pharmaceutical development, manufacturing, and delivery are every day to those patients and their families.