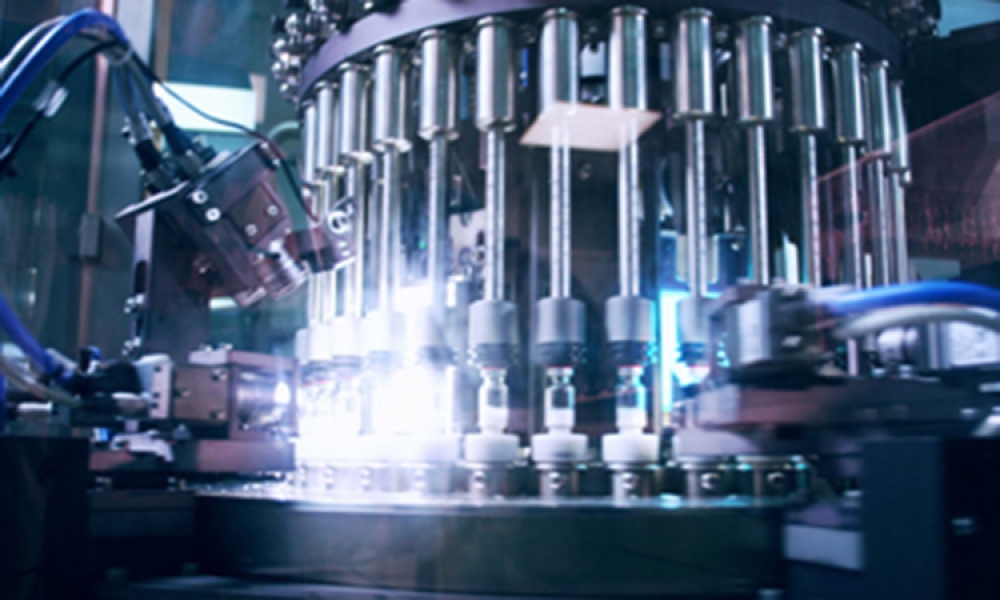
The ISPE Sterile Products Processing Community of Practice (CoP) Steering Committee continues its series of blog posts, where committee members and other contributing subject matter experts dive into the validation and use of essential equipment and processes that drive sterile processing in the pharmaceutical industry. The aim is to provide practical insights that will enhance your...
Per- and polyfluoroalkyl substances (PFAS) have emerged as a major concern in the pharmaceutical industry and beyond. Restricting their use is heavily debated. On the one hand, these “forever chemicals” can pose environmental and human health risks. On the other hand, their unique physical and chemical properties can make them important for pharma and ultimately for helping to enable access to...
Ronald Bauer, PhD and Christina Meissner, PhD from the Austrian Agency for Health and Food Safety (AGES) provided a much-anticipated presentation on the requirements for data management in light of the revised Annex 1 at the 2024 ISPE Pharma 4.0™ and Annex 1 Conference in Rome, Italy in December 2024.
In today’s rapidly evolving pharmaceutical landscape, advancing technologies, shifting regulatory landscapes, and emerging global challenges such as supply chain disruptions, and drug shortages have significantly impacted how we develop, manufacture, and deliver treatments. These factors underscore the critical need for robust validation approaches for computerized systems to safeguard patient...