The proliferation of digital tools available in the pharmaceutical industry, including artificial intelligence (AI) and machine learning (ML), is revolutionizing drug discovery and manufacturing processes.
Think of implementing a digital validation tool (DVT) as being similar to building a new house. There are decisions that need to be made before starting to build a house:
This blog post will discuss the relationship between large systems that manage records at the multi-batch, multi-site level of operation and the data integrity of those records. There are several aspects to consider and they are specific to this type of environment. <
Takeda Austria GmbH has been recognized as the 2024 ISPE Facility of the Year Awards (FOYA) Category Winner for Operations.
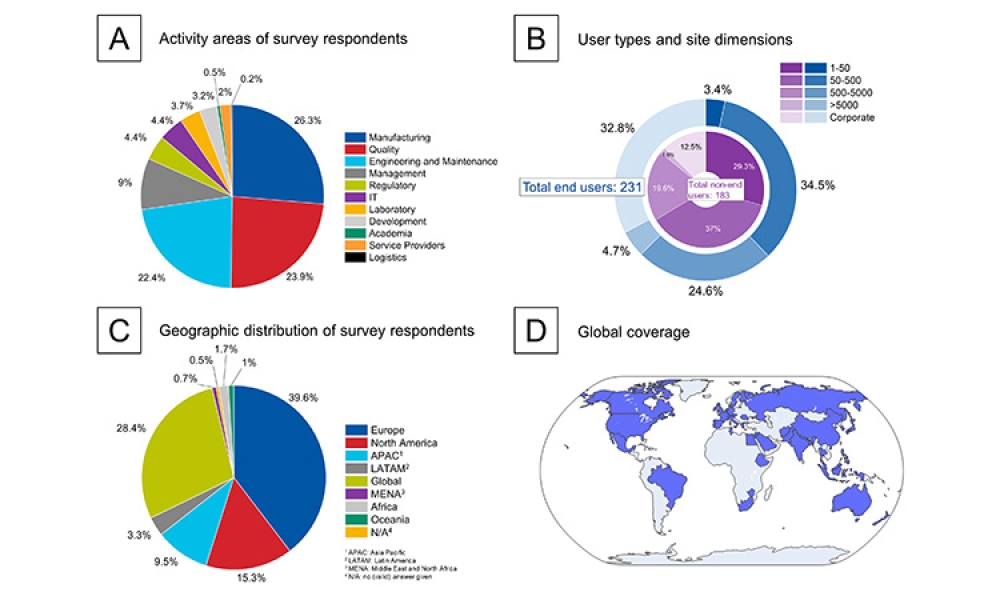
This article summarizes the key findings from the 7th Pharma 4.0™ Survey, conducted in 2023. It explores the demographics of the survey respondents, maturity levels of Pharma 4.0™ adoption, enabling technologies being leveraged, anticipated benefits, and challenges encountered.
Building on last year’s successful collaboration, AstraZeneca is once again stepping up to support ISPE’s Women in Pharma® by sponsoring Women in Pharma® Monday Night Networking Event at the