The Falsified Medicines Directive “introduces harmonised European measures to fight medicine falsifications and ensure that medicines are safe and that the trade in medicines is rigorously controlled.” Such obligatory safety features, legal framework, and record-keeping requirements have arguably imposed stricter controls for manufacturing of medicaments.
Although the pharmaceutical industry has consistently improved manufacturing processes in compliance with good manufacturing practices, there are documented deviations from good practices including the continued falsification of medicines. (Note: The terms “pharmaceutical” and “pharma” interchangeably employ throughout this article.) Disclosure risk assessment techniques in pharma manufacturing typically depend on background knowledge, the behavior of intruders, and the specific value of the data. Often, only heuristic arguments are used, without numerical assessment.
The SPuMoNI consortium comprises two pharma industrial partners—PQE Group and FAREVA’s Instituto De Angeli—and three academic institutions: the Universitat Politècnica de València (Spain), the University of Thessaly (Greece), and the National College of Ireland (Ireland). SPuMoNI utilizes state-of-the-art technologies to support a smarter industry. These technologies include blockchain for end-to-end verification of manufacturing data, quality assurance methods for data integrity compliance, and modern artificial intelligence (AI) and data analytics to smartly extract, transform, and control heterogeneous data sources within the manufacturing processes to better improve big-data quality and process modeling for a smarter industry.
SPuMoNI leverages blockchain technologies to better ascribe and ensure the manufacturing traceability of medicaments. SPuMoNI is particularly timely because blockchain has been proposed to become “a new digital service infrastructure” for Europe. Although blockchain is well-established in the cryptocurrency domain, the systematic application of smart contracts in the pharma industry remains an open problem., Moreover, traceability in manufacturing has traditionally been studied in the food industry, but rarely in pharmaceutical manufacturing, consequently attracting some industry attention.
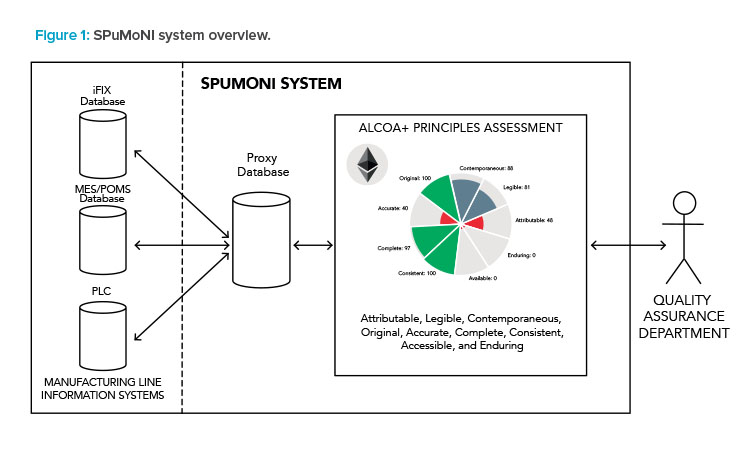
In this respect, ensuring data integrity in compliance with the current Good Manufacturing Practice (CGMP) regulations by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) means ensuring quality assessment of batch reports, audit trails, and system registries in terms of the ALCOA+ principles: attributable, legible, contemporaneous, original, accurate, complete, consistent, accessible, and enduring.
SPuMoNI has produced an innovative scientific approach to systematically establish and ensure constant proof of the authenticity of pharmaceutical manufacturing data and to develop a user-friendly software tool for pharmaceutical officers, following the ISPE GAMP© validation standards, during both the IT development and the use of a qualified IT infrastructure.
End-to-End Verification
Blockchains and smart contracts implement peer-to-peer networks formed by “blocks,” creating a distributed ledger where data from one block can only be altered by modifying all subsequent blocks. In the SPuMoNI system, data are stored within a blockchain as tamper-proof data transactions, ensuring that SPuMoNI datasets remain unaltered with a measurable quality of service. Following General Data Protection Regulation (GDPR) and pharmaceutical industry regulations, SPuMoNI uses its own private Ethereum blockchain network, hosted at National College of Ireland’s OpenStack private cloud, to store data descriptors that should remain confidential with a guaranteed data integrity.