Despite notable initiatives since the early 2000s from regulatory leaders such as ICH, the US FDA, and EMA, the pharma industry has struggled to move on from legacy quality management approaches. The original ICH statements that led to Q8 were intended to modernize pharmaceutical quality systems through a focus on quality risk management and quality by design (QbD). These concepts should have also been a basis for implementing new technologies, as the ICH concepts offer a framework to cope with inherent variability, and allow for a compliant flexibility needed for manufacturing increasingly sophisticated, even personalized medicinal products. However, for pharma organizations and regulators alike, the topic of validation has been a central obstacle to adopting new concepts for quality.
As we look to overcome this obstacle, several questions about validation must be addressed: What would it mean to not write documents in the regulated environment? What do QbD and data integrity mean when applied to a manufacturing facility? Can we move beyond three stages of process validation to defining a control strategy and then continuously verifying that control? We invite you to think about what the answers to these questions might mean for you.
Rethinking What We Do
To illustrate the need for a new mindset, let’s start with a simple example derived from the definition of validation: establishing documented evidence of fitness for purpose. Generally, this principle has been interpreted as a requirement for documentation in either physical or electronic form. For example, during up-front validation testing, documents are used to record how the introduction of a process or system was controlled to ensure it operates correctly.
However, for Validation 4.0, we need to move on from creating historical documents of what was tested to focus instead on real-time verification of product quality by managing specification and evidence data around a process that is in a state of control throughout the life cycle. Standalone documents are clearly not suited to continuous verification, and the masses of documentation created by both suppliers and regulated companies in the name of validation are inefficient, difficult to maintain, and perhaps not auditable.
In the Industry 4.0 world, connected data and systems are subject to rapid and constant change for iterative improvement, and they are now being enabled for autonomous improvement. Instead of relying on difficult to maintain silos of documentation, we look toward digital artifacts managed with appropriate tools that can instantaneously provide reporting and notifications on the state of control. The systems used widely today by agile software developers for multitenant cloud solution providers are a good reference point for Validation 4.0: by adopting the usual tools of software engineers, we can leverage and integrate quality management efforts into our ongoing activities of continuous verification beyond what is possible with static documented evidence.
Data Is the Foundation
Data integrity has been a buzz term for years now. A whole sub-industry has been built around this concept, and yet we still fail to truly embrace what it means and how to implement it. Data is the foundational element of validation and the basis for decision-making. When we consider validation, we need to shift our focus to how we control the data that allows us to make GxP decisions, and look at validation under a QbD lens.
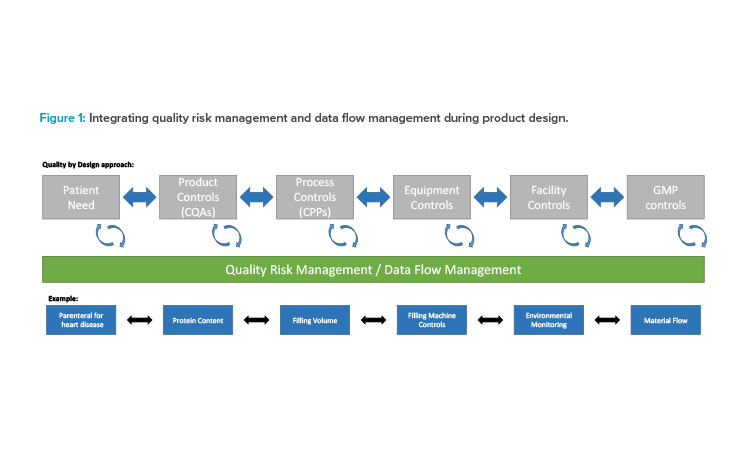
Validation 4.0 is a holistic and integrated approach to validation with process and data flows at its foundation. Let’s follow the product journey that starts with an unmet patient need. To characterize a product intended to meet that need, QbD principles suggest that we begin by defining critical process parameters (CPPs) and critical quality attributes (CQAs). Critical process parameters are defined to control the quality outputs, and we have to understand the critical quality, process, equipment, and material attributes to measure and control them within a defined range of variance that produces a quality product. This design space is an output of product development and the basis for handling inherent variability. It is then expected that as the product moves to actual production, real-time process data will be available and monitored so that the design can be refined. By bringing the QbD concept into Validation 4.0, we further encourage the early definition and use of data points to control and ensure the desired product quality attributes.
A quality risk management approach should be integrated with QbD and applied at the process and data flow level as a part of the design process (Figure 1). First, traditional user requirement specification (URS) statements are replaced by process and data maps (PDM). In Industry 4.0, data are referenceable, used throughout a process, and a basis for making effective decisions. By building a validation model that incorporates the process and key data early in design, we get a head start on defining the associated risks and the needed controls.
Following the initial process and data definition, the focus of validation changes from qualification testing to ongoing and constant assurance that the needed controls are in place and operating correctly. This continuous verification of the process and risk is the primary evidence that the process is in a state of control. By using real-world data to feedback into our process, data, and risk evaluation, we can be assured that our products are constantly manufactured and released based on sound data, and through this model, we can continuously reassess risk conditions and handle inherent process variability.
From Process Validation to Validation 4.0
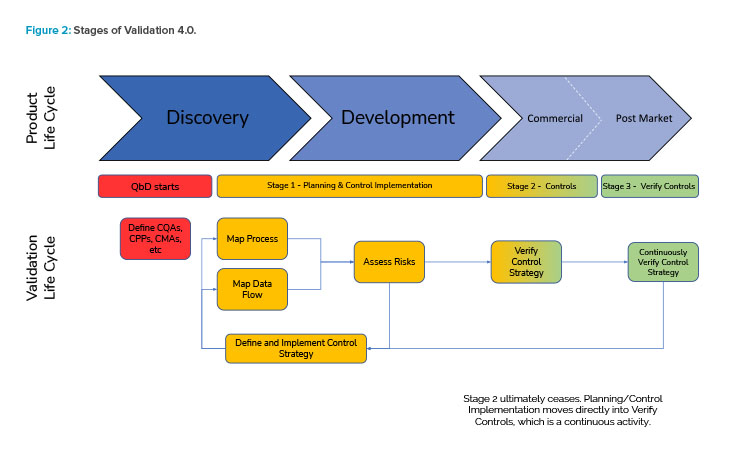
As the US FDA has stated, “effective process validation contributes significantly to assuring drug quality.” Process validation is a series of activities that occur over the life cycle of the product. Table 1 presents how the three stages of process validation can apply to Validation 4.0.
Figure 2 illustrates the holistic view of the Validation 4.0 life-cycle model and how it relates to the product life cycle.