iSpeak Blog
ISPE delivers technical and operational solutions to support across the global pharmaceutical and biopharmaceutical industry in the manufacture of quality medicines for patients.

Featured in this edition of iSpeak Reading Roundup, are the top blog posts from December 2019. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
The growth of single-use technology has revolutionized the bioprocess industry. The implementation of single-use technology has typically been a trial and error exercise for the end users. The main objective of the ISPE Good Practice Guide: Single-Use Technology is to provide the ability to plan, anticipate, and respond to surprises that can arise when implementing this new technology.

Discover the most common violations to avoid for the implementation of a cleaning validation program in today’s industry with product-specific operations, sterile manufacturing, and how to organize pre-approved strategies. In addition, solutions to these common pitfalls are featured in this article.
Falsified Medicines Directive 2011/62/EU was published by the European Parliament on 08-Jun-2011. The Directive was implemented to increase the security of the manufacturing and delivery of medicines across Europe, protect patients and prevent falsified medicines from entering the supply chain.

The formal announcement and certification of the ISPE Mexico Affiliate took place at the recent 2019 ISPE Annual Meeting & Expo in Las Vegas, Nevada. The creation of the ISPE Mexico Affiliate was promoted by a group of enthusiastic expert industry professionals from various companies considered as benchmarks in the pharmaceutical and biopharmaceutical industry sectors in Mexico.
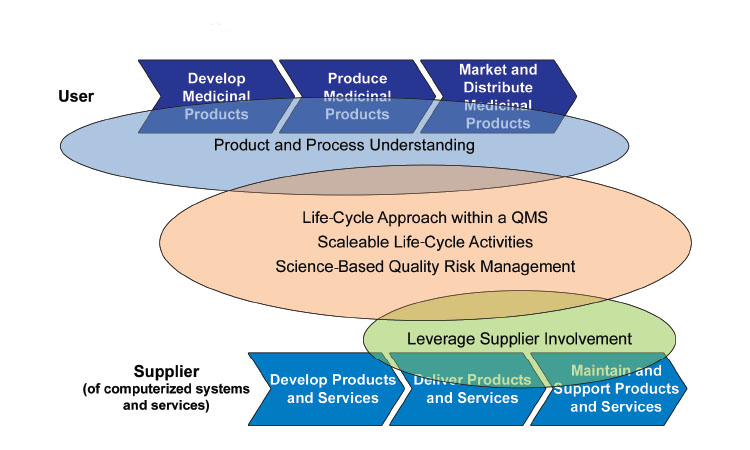
The GAMP® Global Leadership strongly supports a patient-centric and quality risk-based approach to the assurance of computerized systems. Explore the incorporation of innovative computerized technologies and approaches that advance pharmaceutical production to support product quality, patient safety, and data integrity.
In the pharmaceutical industry, disaster recovery is not just about protecting business continuity; it is about safeguarding patient safety, data integrity, and the entire supply chain. The increasing complexity of global pharmaceutical operations exposes companies to a broad spectrum of risks, ranging from man-made disasters such as geopolitical conflicts to natural disasters that can cripple...
ISPE’s Q12 Implementation team, a working group under the auspices of ISPE's Product Quality Lifecycle Implementation (PQLI)® committee, continued their series of training events with a well-attended course delivered to Singapore’s Health Sciences Authority (HSA) in November 2024.