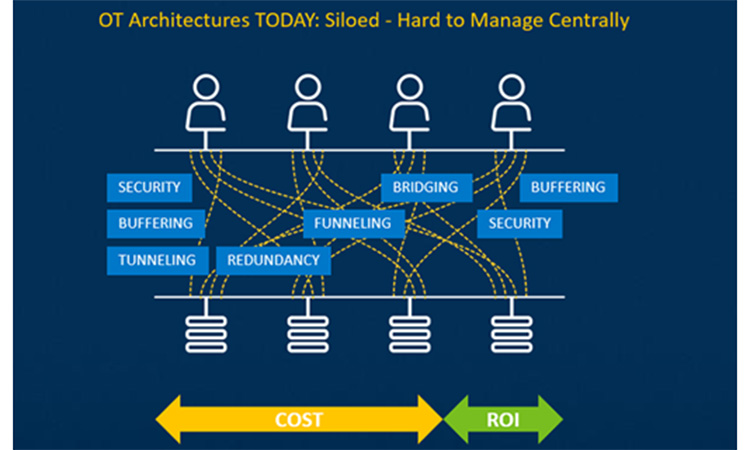
The biggest organizational cost to this inefficient architecture is more than these logistical concerns. The time required to gather and make sense of data using this type of system limits the speed of innovation. An Operations Data Hub - an enterprise level solution with secure and governed data access across the business, a simple user interface to quickly define and evaluate operations improvements, and tools to easily deploy new operations solutions, can accelerate innovation and directly impact profitability.
What IT/OT challenges should be addressed?
Below we detail four pharma OT and IT challenges which an Operations Data Hub needs to address: scalability, security, ease of use and compliance.
Additionally, the system needs a mechanism to facilitate distribution of data between two different physical networks separated by DMZ (Demilitarized Zone) which shall act as a bridge between OT & IT networks to ensure secure & seamless data transfer.
Additionally, the system needs a mechanism to facilitate distribution of data between two different physical networks separated by DMZ (Demilitarized Zone) which shall act as a bridge between OT & IT networks to ensure secure & seamless data transfer.
- Scalability – an enterprise native solution
As pharma companies look to increase agility, pharma manufacturers need to accelerate responsiveness to changes and integrate operations with business data to drive better decisions. An enterprise approach not only provides better data but can also assist with leveraging improvements between plants. Enterprise challenges can be categorized by the 3V’s of big data:
- Variety: ability to ingress a wide range of data (such as Time series over OPC, SQL data, Files, multimedia, MQTT, Kafka, Modbus etc.) from external sources in a managed fashion (Ex OPC-DA) or Unmanaged fashion (Ex. WebAPI).
- Velocity: ability to manage network bandwidth while streaming real-time data from external data sources or transporting historized data, without over-straining the system.
- Volume: ability to scale as the complexity of infrastructure requirements and the amount of data to store grows. Pharma/Biopharma companies prefer a single system across a global manufacturing network, which further exacerbates this challenge.
- Secured by default
When the FDA determines a manufacturer violated manufacturing practices, including ALCOA, they issue a Warning Letter. A close-out letter is issued after the manufacturer takes corrective action to address the violations contained in the Warning Letter. Hence it is important that any Operations Data Hub used in Pharma must have native security features, to ensure data integrity which is essential to the safety, purity, quality, and efficacy of drug products including:
- Encryption of intra component (software) communication during transit of data
- Transport layer authentication (Ex. SCRAM-SHA-256 Authentication, SCRAM-SHA-1) during bidirectional communication with data archiving system or third-party applications
- Active directory-based authentication & role-based access model for user access management
Additionally, the system needs a mechanism to facilitate distribution of data between two different physical networks separated by DMZ (Demilitarized Zone) which shall act as a bridge between OT & IT networks to ensure secure & seamless data transfer.
- Ease of implementation and maintenance:
Corporate IT departments are stretched thin managing the new workload resulting from digital transformation. They need a solution that is easy to install, scale, and maintain.
- Many enterprise OT data solutions can take days to months to deploy. Future systems need to be faster, minute to hours, and easier to deploy for pharma companies to accelerate their digital transformation.
- In many cases, it is a challenging and time-consuming effort to upgrade to the latest version of the OT data system or install patches to every server, workstation, and client machine. Systems should auto update features to assure that whole systems, whether remotely connecting or otherwise, have the latest version of the client software or patches without manual downloads.
- The cost of current enterprise OT data solutions includes hardware (i.e., Servers, Workstations/PCs), operating system (OS), and the labor to support the enterprise system. A centrally managed Operations Data Hub can reduce costs in all areas, simplifying configuration, monitoring, license management and supervision of the system as well as the status of single components.
- Regulatory (GxP) Compliance:
Regulations in the pharma sector require that any event involving the creation, modification or deletion of records relating to manufacturing is recorded, maintained and retrievable. A software application must produce an audit trail to be compliant per FDA regulations (21 CFR Part 11) and EU Regulation (EU Annex 11, Section 12). This means each software application generates its own audit trail. The Operations Data Hub must be compliance-ready itself (recording events) and it should be a single source of truth for audit trails.
Join the webinar to learn about a new approach to enterprise OT data management.
Register Now!
To learn more about these challenges and how they are being met by pioneers in pharma, join the upcoming webinar, “Progress Your Pharma 4.0 Journey by Solving Industrial DataOps Challenges” on March 9th, 2023 at 10 am EST/4 pm CET