The applications are endless as it entirely depends upon the current issues, imagination, and alignment of plant stakeholders to target the specific challenge. It is critical that the RTLS journey should keep the bigger picture in mind while looking for low hanging fruits. This will ensure an easier buy-in from the program sponsor. RTLS should always be considered as a critical element in Life Sciences’ Smart Factory journey as it can work as an enabler to improve operational excellence and accelerate lean operations.
Introduction:
Real Time Location Systems (RTLS) are used to track the exact location of any object. On the manufacturing shopfloor, RTLS can provide real time location of any object with different levels of granularity as exact location identification is required at various stages when an object continues to move from one process to another. A variety of sensors can be used for RTLS based upon specific needs. It also depends upon the object being tracked, the factory layout and the location accuracy needed. RTLS can be very useful in contributing to the overall operations excellence journey and can aid in lean manufacturing. Generally, RTLS solutions can be simple or very complex depending upon the issues which must be resolved. Typically, an RTLS solution would include various sensors, gateways, and various component of RTLS software which would provide the exact location. Accuracy depends upon the various RTLS solution elements selected for the purpose.
Problem Description:
Location tracking is very critical in day-to-day operations in pharma/bio-pharma manufacturing as it directly impacts the plant efficiency, material wastage, streamlining of operations, asset usage and regulatory compliance. We have seen that the shopfloor personnel understand the issues faced and time spent on tracking raw material, intermediate material, finished products, consumable parts and controlling the movement of equipment like chromatography columns, membrane etc. to meet regulatory requirements in core and tertiary areas be it the warehouse, the staging areas, the production areas, or the temperature-controlled areas. Hence, it’s imperative to identify the location in real time to achieve lean manufacturing.
Key Trends in Life Sciences Industry:
Most life science manufacturers have started evaluating RTLS use in plants while leading manufacturers have started implementing RTLS solutions for operational excellence.
A case in point could be a situation where RM location and material availability was entered in the ERP manually which was then causing RM availability issues on the shopfloor. By implementing RTLS solution, pharma manufacturers were able to locate material and availability of this very material which is being used to create bookings in the ERP system automatically.
Similarly in another case, RM was re-dispensed as material was moved to other locations because of production and maintenance activities leading to significant waste. By implementing RTLS, operators were able to check the exact location of the material in production area prior to requesting for more material. Also, RTLS helps to reduce the scanning operations and hence optimizes human effort.
Potential RTLS Use Cases in Biotech Manufacturing:
Below is an illustrative list of use cases in biotech manufacturing where RTLS solution can be leveraged:
- Consumables and material tracking, such as media and buffer tote location, RM from warehouse to dispensing to various stages in production area which would help in lean operations.
- Tracking of costly items like chromatography columns and membranes. Knowing when the items are in use for production vs. in a hold condition can enable better production planning, inventory management and efficient procurement.
- Tracking of API bottles from filling stations to conditioning area and then conditioning area to freezer and can reduce the use of paper logbooks.
- Parts clean status tracking by utilizing RTLS transponders on carts or equipment
- Time out of refrigeration tracking of samples from manufacturing area to lab, ensuring the integrity of samples.
- Auto-population of parts in MES used in set-up when the cart/totes enter to production area.
- Tools tracking on shopfloor to manage movement from pre-viral to post-viral areas.
RTLS Execution Journey:
RTLS journey should include not only the pointed solution which may resolve current issues but should be defined while keeping future requirements in mind. Hence it should include the following steps:

The best approach while moving forward with RTLS is to start with an assessment exercise to identify prioritized use cases, architecture requirements to meet those use cases, budget requirement and scalability to meet future use cases requirement. While a point RTLS solution can be implemented very fast but may not be the right fit to meet future expansion needs. Hence RTLS infrastructure design should keep all these things in mind while meeting the current requirements. Factors like third party applications interfaces, support to IoT communication protocols like MQTT, OPC UA, etc., cabling requirements, wireless operation, regulatory compliance can play a big role while deciding the RTLS product.
A Proof-of-Concept (PoC) could be the right starting point to understand the benefits and get buy-in from business stakeholders. This phase will check the fitment and feasibility of solution in the specific manufacturing environment. Once POC is deployed successfully, a full-fledged RTLS project based upon prioritized use cases can be initiated.
Typical RTLS solution would include RTLS hardware (like RTLS sensors, Gateways, Power supplies, Network switches, Antennas etc.) and various RTLS software components. Hardware components would depend upon the expected coverage area, location accuracy, obstructions and number of items which need to be tracked.
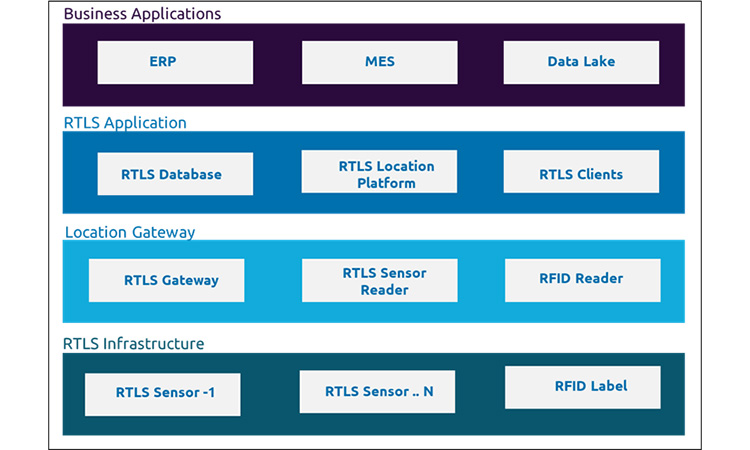
A Typical RTLS use case can be implemented in a few weeks’ time and can extend to many months depending on the scale and complexity required.
Conclusion:
RTLS solutions could help to meet operational excellence goals in biotech manufacturing. It would depend upon the pain point of a particular plant and would vary from one plant to another even for the same manufacturer, as existing business and IT ecosystems could entirely be different in two plants. Best approach is to identify key issues related to material/asset/compliance tracking and check the fitment of RTLS and different components of RTLS required along with the potential return on investment from the solution. One thing is for sure, RTLS must be a critical element in the Smart Factory journey.







