ISPE Holds Expert Xchange: Regulatory Summit on Modernizing Inspections

As we are all aware, the COVID-19 pandemic has had a unique, world-wide effect on the pharmaceutical industry as well as health authorities. At the height of the pandemic, and continuing as it appears to wane, health authorities have not been able not carry out normal, in-person inspections of pharmaceutical manufacturing sites. However, in the ”Age of Covid,” using the Internet and associated technologies have made remote “virtual inspections” possible.
As virtual inspections continue and some in-person inspections resume, regulators and manufacturers are determining the efficacy of remote inspections and how they can be improved for future use. Given the lessons learned over the last few years, both the industry and regulators are also seeking how to use the virtual inspection experience to determine if “modernizing” inspections using remote technologies is feasible.
On September 14, ISPE held a virtual Regulatory Summit on Modernizing Inspections. The event was aimed at understanding the current status of reliance as applied to remote inspections and what future steps might be given recent experiences with reliance as applied during the pandemic. On-site inspections are preferred and are considered the “Gold Standard” approach to inspections from a practical and legal perspective. The Summit explored situations where proper planning, coordination, and communication are achievable and asked how can Remote Interactive Evaluations/Distant Assessments (or other remote/virtual approaches) become effective tools for playing an important part in future regulatory inspections?
Considering that virtual communication tools such as Zoom and MS Teams are now fully integrated into society and are commonly used across the pharmaceutical industry, how can these tools play a future role while maintaining forward progress on the development, manufacture, and distribution of critical therapeutics for the public?
Presentation Highlights


The session began with a presentation by Susan Laska, who offered PIC/S’ perspective on Mutual Reliance by noting that it was PIC/S’ 50th anniversary. She explained that PIC/S is a cooperative arrangement representing fifty-four participating regulatory authorities The PIC/S mission is to lead the international development, implementation, and maintenance of harmonized Good Manufacturing Practice (GMP) standards and quality systems of inspectorates in the field of medicinal products.
She said that instituting remote inspections during the pandemic has been challenging, but that carrying out “desktop” audits are “nothing new,” as Canada, Australia, and the World Health Organization (WHO) have been conducting them for many years. Laska noted an April 2019 meeting where Health Canada introduced a tool for desk top audits to conform to GMP compliance requirements. She also cited a 2020 PIC/S seminar in Finland where presenters demonstrated aspects of conducting remote inspections.
Further, in June 2021, PIC/S carried out a joint, two-day webinar regarding best practices for remote inspections. Attendees agreed that remote inspections cannot replace in-person inspections, which she referred to as The Gold Standard.
“Facilities need to have the technology to participate, and Inspectors have to work longer hours,” she said, adding that remote inspections may take two times more time than required for conducting in-person inspections.
Finally, Laska referred to the PIC/S working group’s focus on the conduct of remote inspections and their efforts to develop a guidance on remote assessments that will not duplicate efforts made by other organizations. That work involves developing training materials, shared definitions and harmonizing various terminology.
“There have been robust discussions regarding harmonizing terminology,” she said and concluded by saying that the working group will soon be sharing the fruits of their work.

The WHO perspective on Mutual Reliance was offered by Stephanie Croft. Croft spoke on WHO’s changes in regulatory focus since the pandemic, inspection challenges during the pandemic, and what those challenges meant for the future of regulatory systems.
“Along with a change in focus, we need to unite, collaborate and cooperate,” said Croft.
Croft suggested that the work done during the pandemic highlighted the importance as well as the role and value of collaboration and cooperation of outside organizations and the need to share best practices and experiences. The pandemic also highlighted the need for local production of medications and greater access to medications, especially those health products related to the pandemic, but that there were challenges in establishing the GMP status of some under-resourced sites. She noted that in some sites there were “huge gaps in regulatory capacity in terms of human and financial resources.”
She reviewed WHO’s Global Benchmarking Tool (GBT) which can help determine site performance maturity.
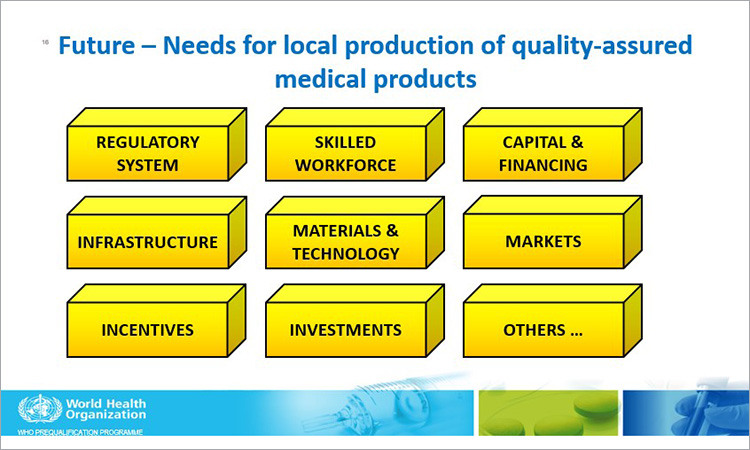
She said that WHO is making efforts through its Global Benchmarking Tool (GBT) to help with continued improvement on the regulatory system and that engagement in WHO’s Regulatory Systems Strengthening (RSS) could help regulators evaluate national regulatory systems by applying WHO’s evaluation tools to generate evidence of regulatory system performance and facilitate implementation of Institutional Development Plans (IDPs). She also identified the need for local production of quality-assured medical products.
Croft closed by emphasizing that regulatory systems should be science-based, respect international standards and best practices, and adopt an approach that focuses on what cannot be done by others while leveraging the work of other trusted regulators and institutions.
Brendan Cuddy, EMA, Stelios Tsinontides, US FDA, and Karl Cogan, (HPRA), discussed the International Coalition of Medicine Regulatory Authority (ICMRA)’s Regulatory Collaboration Pilot projects.
Kogan highlighted the Pharmaceutical Quality Knowledge Management System (PQ KMS) pilot project and explained how it grew out of a 2019 reflection paper that laid out the potential for developing a regulatory knowledge system as well as sharing facility information between regulatory agencies with the goal of improving and harmonizing reliance in a “streamlined” regulatory system. He discussed the origins of the PQ KMS concept and the current efforts of the working group as they dealt with a “massive body of work.”
Kogan introduced the pilot effort to develop hybrid inspections by developing an assessment framework to collect information. One of the goals was to understand differences and why they existed.
Cuddy also discussed the concept behind Collaborative Hybrid Inspections Pilot (CHIP), highlighting its aims and benefits to stakeholders. He said that collaborative work on pilots of hybrid inspections is on-going and is part of their framework involving “boots-on-the-ground” aspects of hybrid inspections when there are two or more authorities conducting the inspection.
“We hope to develop the feasibility of hybrid inspections generating data and identifying opportunities for implementation,” said Cuddy, adding that there should be benefits to all stakeholders as the effort may shorten assessment and approval times. “However, all of the authorities must agree on the deficiencies and develop a single action plan.”
He explained that the lead inspectorate will maintain contact with the facility and that the lead inspectorate and remote inspectors will develop the CGMP deficiencies list and the CAPAs will be reviewed by all participating authorities.
The pilot will initially involve small molecules and biologics but will eventually broaden to include all critical medications.
Tsinontides said that following the Collaborative Hybrid Inspections Pilot 2021 workshop, they hope to implement the pilots on Collaborative Hybrid Inspections in 2023. He explained the Inspection Pilot Application form and highlighted the facility-specific information that would allow a facility to take part in the pilot and where to submit the application.
“Despite the challenges and constraints, we are committed to working together to find commonalities and convergence and bring safe and effective medicines to patients world-wide,” concluded Tsinontides. He added that they hoped to share their findings by the end of 2023.
Deborah Smith, Janssen, asked attendees to think about what does “modern” mean? And, considering that on-site inspections are considered to be “The Gold Standard,” what does that phrase mean?
Smith continued by saying even when there is no pandemic “we are always interested in finding ways to be more agile and efficient” and offered her company’s recent experience and an industry perspective on on-site, virtual, hybrid and desktop inspections.
Having had a virtual inspection during the height of the COVID-19 pandemic, followed by an on-site inspection at the same manufacturing site a short time later, Smith explained some of the difficulties and challenges, as well as pros and cons, that virtual inspections entail as compared to on-site personal inspections.
Smith noted that virtual inspections require more of inspector’s time as well as longer preparation time for the facility. To facilitate good virtual inspections, company’s need to have an increased number of staff members – acting as the “production crew” - adept at using multiple platforms and the best technologies.
“However,” said Smith,” when the virtual technologies are working well, inspectors have a front row seat. The difference is like having a front row seat watching a football game on TV versus being in the stands at the game. You can see much more of the game on TV.”
Among the issues on the “cons” side is that the subject matter experts (SMEs) were unable to travel and were not on-site.
“Virtual inspections need equal value with on-site inspections,” she concluded, adding that she is “not a big fan” of desk-top inspections because they eliminate all two-way interaction, and interactivity needs to be preserved. She concluded by saying that the terms “virtual,” “hybrid,” and “desk-top” need further defining.
Panel Discussion
Moderators Millili and Higgins introduced the discussion panels participants and led the discussion of questions submitted during the conference from attendees. The presenters from the first part of the session were joined by Anderson Alves da Silva, Specialist in Health Regulation and Surveillance, ANVISA, and Jason Treese, Vice President of Quality, Bristol-Myers Squibb.
Highlights of the discussion are presented below.
1) Has PIC/S Remote Assessment Working Group considered redefining the term “inspections” and broadening its scope?
Laska said that PIC/s has had robust discussions regarding term definitions and that we all know that the future will include remote and hybrid inspections, adding that there is a tremendous amount of work coming out of the Working Group that we should see soon.
2) Would digitalization help WHO to conduct remote inspections? What needs to be done to make them possible?
Croft said that digitalized systems would help to conduct remote inspections, but she cautioned that trying to conduct remote inspections in low-and middle-income countries, like the sites that WHO is likely to inspect, may have difficulty providing the necessary technology and the Internet access that makes remote inspections possible.
3) For collaborative hybrid inspections, will the regulated party receive one or multiple reports outlining contraventions/deficiencies or just one report? In addition, how will regulatory differences/nuances be addressed to meet the requirements of the respective regulatory authorities and who will follow-up to ensure implementation of corrective actions – the lead RA or all of the RAs?
Cuddy said that there will be a single list of deficiencies and recommended CAPAs that all of the regulatory authorities involved will sign off on. However, there may be a lead regulatory authority. When collaborative teams issue their reports, there may be legal constraints imposed upon them by national legislatures. Also, there may be more than one report, but just a single list of agreed upon deficiencies. Enforcement and follow-up may be up to an individual health authority.
4) For your on-site inspection that occurred after the virtual inspection, was the on-site inspection more focused on aspects that could not be covered, or were challenging to cover, during the virtual inspection? Or was it a full on-site inspection? Was it the same inspectors who conducted both the remote and on-site inspections six months apart?
Smith said that there were numerous challenges in preparing for a remote inspection over and above when they had an on-site inspection, including considerations for portable technology, Wi-Fi availability and having Wi-Fi “dead zones” in manufacturing areas, time zone differences, and the need for the site staff to practice in advance.
There was some duplication of work when the same inspectors carried out a virtual inspection and then conducted an on-site inspection just a few months later.
5) Since paper record keeping is still prevalent and creates a barrier to remote inspections, are the agencies providing an opinion on the moving to digital tools such as electronic batch records and review by exception to better support off-site reviews?
Stellios Tsinontides said that they are examining a proposal from industry on the better use of paperless information but setting no requirement for electronic batch records.
Disclaimer
This article contains an abridged, unofficial summary of presentations and discussion during a virtual event on 14 September 2022. This content has not been vetted by any regulatory agency, represents an informal and brief synopsis of regulators’ views, and does not represent official guidance or policy of any regulatory agency or authority.









