4. Pursue responsible transparency. Stakeholder interest in greater transparency of supply chain or quality maturity information has been steadily increasing to support market-wide predictive analytics. Predictive analytics could more rapidly and accurately identify market vulnerabilities to support earlier interventions or set the stage for market incentives for manufacturers to generate more reliable supply. Because of these potential benefits, new regulatory expectations have been developed or are under consideration globally. ISPE has had a longstanding commitment to constructive health authority engagement. ISPE continues to support industry-health authority interactions when appropriate to optimize risk management for product availability and optimize supply disruption mitigation, as needed. However, transparency of the complex pharmaceutical supply chain without sufficient context may be problematic. Therefore, ISPE recommends that pursuit of further data exchange, and in particular any initiative to increase pharmaceutical data publication in public forums, should be carefully considered to ensure unintended consequences such as, panic-buying or hoarding, loss of patient trust, impact to supply chain security, proprietary data infringements or unnecessary administrative burden are avoided.
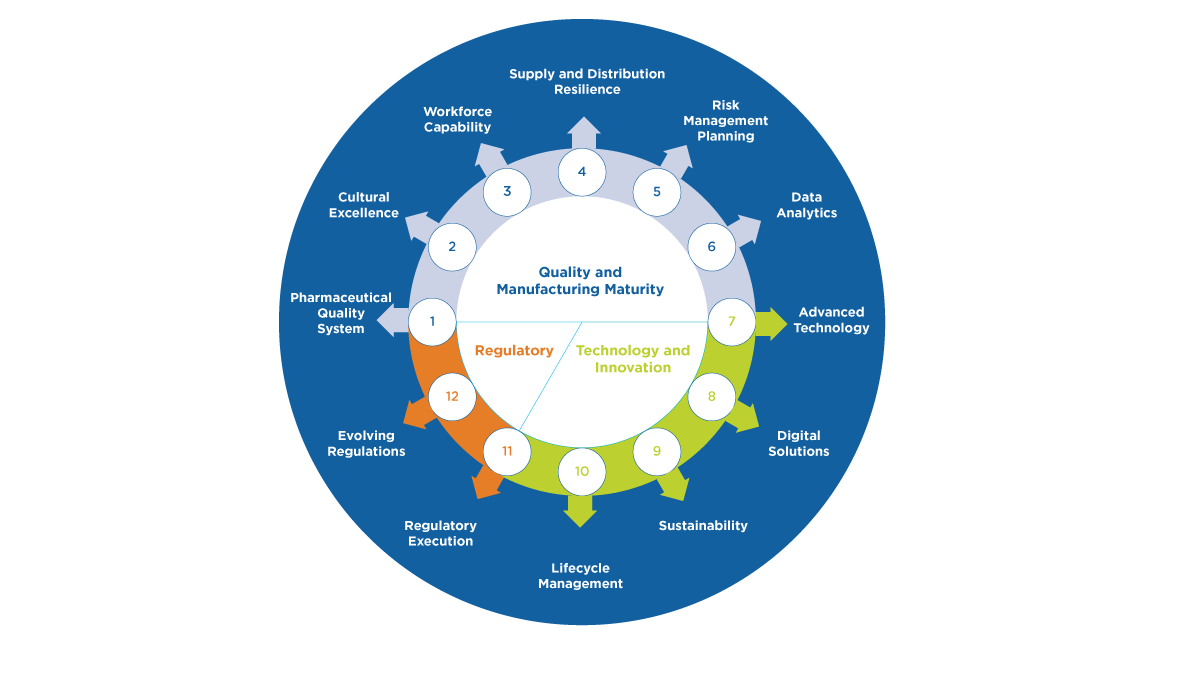
5. Technical advancement is foundational to supply resiliency. ISPE continues to generate information exchange platforms for advancing expertise and progress in various areas that may be transformational for supply resiliency, including, data analytics, digital solutions, advanced technology, and lifecycle management.
6. Market dynamics and related geo-political considerations are outside the scope of ISPE focus and may have a significant impact on product availability because of the challenging demand fluctuations they may generate. ISPE’s scope includes the technical, quality and regulatory aspects to supply resiliency and drug shortage prevention. ISPE input to the evaluation of potential solutions to drug shortages and supply resiliency challenges beyond these areas of expertise may be limited. However, ISPE does continue to provide guidance and platforms for discussion regarding how industry may improve risk management, overall quality maturity and drug shortage prevention performance, because these capabilities will provide the agility and ability to mitigate the impact of unexpected market dynamics or geo-political generated issues as quickly as possible.
7. Investing in talent is critical to our success. Considering the post-COVID workforce dynamics, ongoing depletion in trained pharmaceutical experts, and focusing on future resources needs, it is evident that a renaissance in how the industry attracts, develops and retains talent is essential for supply resilience. Additionally, the industry needs to consider what new roles may be needed to lead holistic supply resiliency at an enterprise level within manufacturing companies. The ISPE Emerging Leaders program and ISPE Workforce of the Future initiative both support this important evolution.
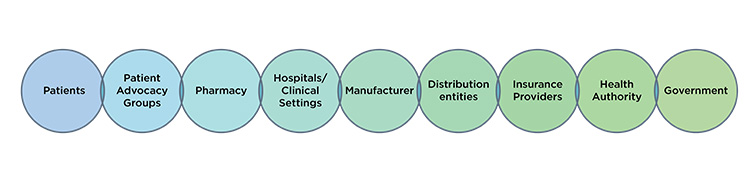
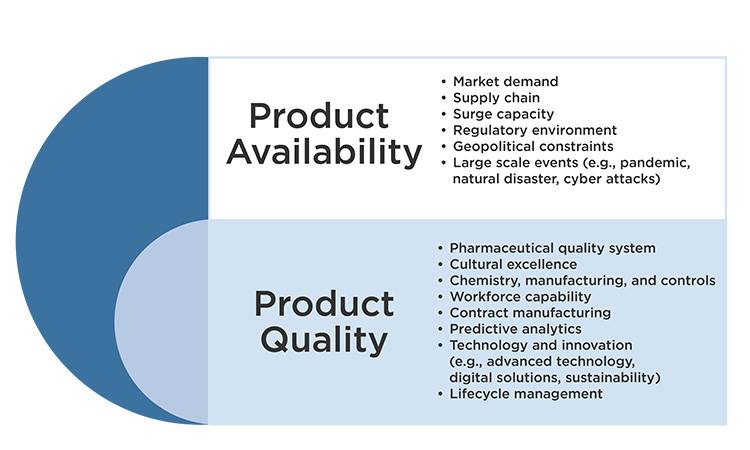
ISPE’s observation is that supply resiliency is a journey. How a manufacturer may support continued supply throughout a product lifecycle may vary. How manufacturers may support continued supply over time in relation to evolving regulatory requirements may vary. How manufacturers may support continued supply throughout the global marketplace may vary. Furthermore, no one is working in isolation. All global stakeholders noted herein (Figure 1) have an important perspective and contribution to supply resiliency outcomes. Only when all global stakeholders approach supply resiliency with a holistic mindset will we serve every patient to the best of our ability globally.
Disclaimer:
iSpeak Blog posts provide an opportunity for the dissemination of ideas and opinions on topics impacting the pharmaceutical industry. Ideas and opinions expressed in iSpeak Blog posts are those of the author(s) and publication thereof does not imply endorsement by ISPE.