In the bustling city of Boston, amidst the vibrant biotech community, the 2024 ISPE Biotechnology Conference will gather industry leaders and innovators for a pivotal set of discussions on Track 4: Lifecycle...
Christoph Herwig, PhD

Related Articles
Are you interested in developing a new bioproduct and attracted by the incredible potentials of new modalities like ATMPs, Cell and Gene Therapies, AAVs, oligonucleotides, multi-specificity antibodies, mRNA vaccines, and more? Despite the appeal of these new modalities, there is a significant risk of failure due to the many unknowns associated with these new technologies.
This second of a two-part series explores digital transformation and digitalization in the biopharmaceutical industry with information about how data science enables digitalization along the product life cycle. (Part 1 was published in the March-April 2021 issue of Pharmaceutical Engineering.
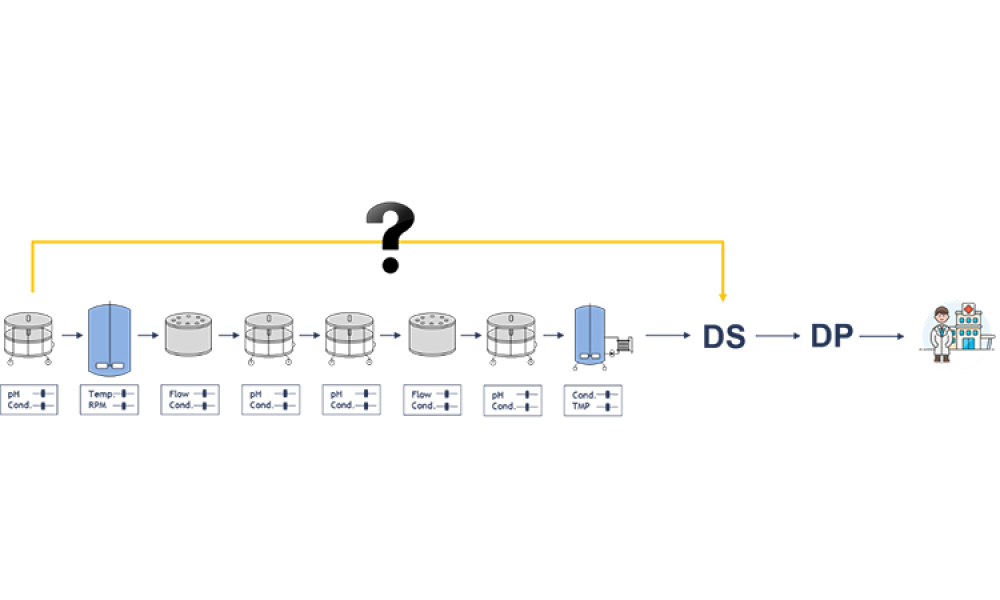
Digital transformation and digitalization are on the agenda for all organizations in the biopharmaceutical industry. But what are the main enablers of intelligent manufacturing? We hypothesize that data science–derived manufacturing process and product understanding is the main driver of digitalization in the bioprocessing industry for biologics manufacturing. In this article, the first of a...
Process validation (PV) aims at reassuring a manufacturer of constant product quality. Moreover, it is a regulatory requirement to achieve licensure of a pharmaceutical product Failing to efficiently plan and execute activities here leads to increased time-to-market. Statistics play a pivotal role here, as is shown by the fact that in the 22 pages of the latest FDA guidance document on process...
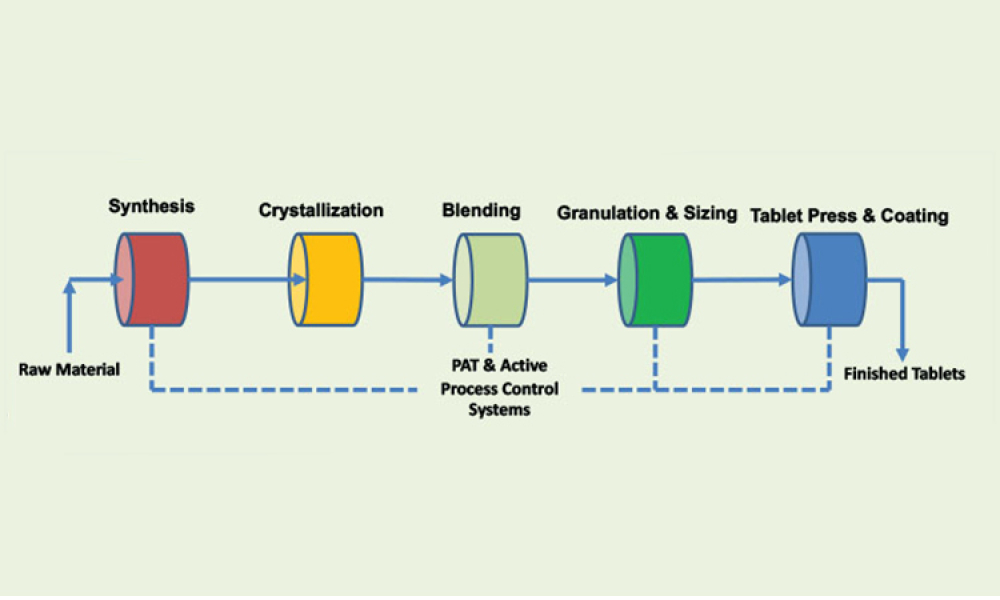
Process analytical technology (PAT) is perceived as the main enabler for a robust control strategy with continuous manufacturing (CM) because process analytical technology can aid in implementing continuous manufacturing throughout the entire life cycle. This article discusses quality and regulatory hurdles in the life cycle of a process analytical technology application—including model...